Batch Distillation Rayleigh Method
A batch of crude pentane contains 15 mole percent butane and 85 mole percent pentane. It is added to a still and heated at atmospheric pressure. How many moles are left in the still when the remaining charge in the still is 97% pentane?
Initial conditions – work on the basis of 1 mole, i.e. no = 1 mole. xo = 15% = 0.15
At the end, x = 3% = 0.03. What is n? Use the Rayleigh equation.
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
Batch Distillation Rayleigh Method
How to solve the problem
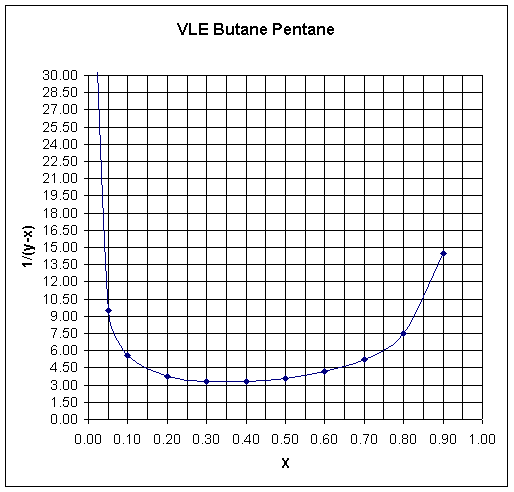
Draw vertical lines at x = 0.03 and x = 0.15. Count the number of squares enclosed toget 11 squares. Each square has an area = 0.05x1.5 = 0.0075. Therefore, ln(no/n) = 11x0.0075 = 0.825
Compare this to the integrated equation which is:
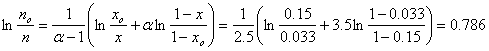
Enter the numbers for each term in the equation to get ln(no/n) = 0.786.
ln(no/n) = 0.825 means that no/n = exp(0.825) = 2.28 means that n = 1/2.28 = 0.438 mols
ln(no/n) = 0.786 means that no/n = exp(0.786) = 2.28 means that n = 1/2.19 = 0.455 mols
There are 0.455 moles left in the still. We started with 1 mole. The still is 97% pentane.
Compare to Unit Ops, McCabe Smith, 6th Ed., p702, example 21.9.